
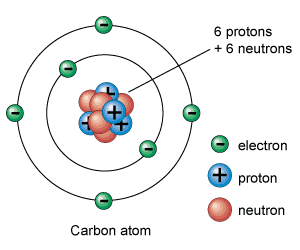

It requires a great deal of energy, however, to break an atom into its component parts. The word atom comes from a Greek word meaning “indivisible.” Since the end of the 19th century, however, it has been known that atoms are themselves made up of smaller particles. Scientists long believed that atoms were “elementary particles” that had no discernible structure and could not be broken apart. It takes more energy to break the chemical bonds that hold atoms together in molecules.
Separating large groups of molecules is relatively easy. Each water molecule is in turn made up of two hydrogen atoms and one oxygen atom that have been combined chemically. A glass of water, for instance, contains an extremely large amount (about 8 × 10 24) of water molecules. Since atoms, ions, and molecules are very small, the bulk matter of everyday life consists of large amounts of these components. Matter is made up of molecules, atoms, and ions (electrically charged atoms or groups of atoms), so atoms are basic components of matter. Atoms also join together chemically to form molecules. Elements are made up of only one type of atom-gold contains only gold atoms, and neon contains only neon atoms-but other substances are mixtures of different kinds of atoms. More than 90 types of atom exist in nature, and each one forms a different element. An atom is the smallest piece of matter that has the characteristic properties of a chemical element, such as hydrogen, oxygen, calcium, iron, gold, and neon. The tiny units of matter known as atoms are the basic building blocks of chemistry.


 0 kommentar(er)
0 kommentar(er)
